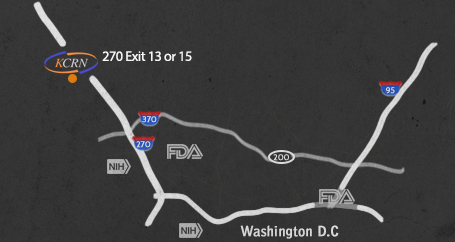
About Us
Giving Flexibility to Early Phase Clinical Trials!

Proactive, motivated, and focused
KCRN Research is committed to providing clinical and regulatory services that are specifically tailored to meet your needs. We are focused on addressing the need for an efficient and cost-effective path to drug development. Our unique approach shows that we strive to share the common goal of a swift and smooth entry into the clinical trial process with our clients. In other words, we make your success our highest objective.
We consider ourselves an extension of your team and will engage in your projects with an attitude that is aligned with yours. In other words, we operate with a Sponsor-centered mindset.
More Information about KCRN
Company History
From the beginning, we’ve had a Sponsor-like mindset as our focus. The Founder of KCRN, before starting the company in early 2012, has had extensive experience on the Sponsor-side of the industry as a clinical and regulatory project manager within various pharmaceutical companies. As the primary contact with the CROs involved in his projects, he gained insight on the limitations of the Sponsor-CRO relationship. He experienced first-hand how often CROs sidestep the partnership during delays and readily take advantage of any opportunities to increase the budget. And he saw the need for a CRO that can deliver both quality and cost-effectiveness together in a way that provides an overall satisfying experience for everyone involved.
Utilizing his expertise, he then set out to create a firm that evolves the Sponsor-CRO relationship to provide the highest level of quality service while preserving cost-efficiency. Today, we operate with that same belief; keeping the interests of the Sponsor in mind within every step of our operations by applying our unique knowledge and proficiency.


Our Expertise
With the KCRN methodology of operations, we are ready to handle any early phase drug development project that might come our way, regardless of the indication of the drug. Our team is committed to ensuring that your project is done with the highest quality in capable hands.
| Indication | Pre-IND | IND | Phase 1 | Phase 2 |
|---|---|---|---|---|
| Solid Tumor | ✓ | ✓✓✓✓✓✓ | ✓✓✓ | |
| Myelodysplastic Syndrome | ✓ | ✓ | ||
| Breast Cancer | ✓✓ | ✓ | ||
| Glioblastoma | ✓ | ✓ | ||
| Hepatocellular Carcinoma | ✓ | |||
| Lung Cancer | ✓✓ | |||
| Head and Neck Cancer | ✓✓ | ✓ | ||
| Ulcerative Colitis | ✓✓✓✓ | ✓✓ | ✓ | |
| Alzheimer’s Disease | ✓✓ | ✓✓✓✓ | ✓ | ✓ |
| Parkinson’s Disease | ✓ | ✓ | ✓ | |
| Major Depressive Disorder | ✓ | ✓ | ||
| Amyotrophic Lateral Sclerosis | ✓ | ✓ | ||
| Cerebral Palsy (Non-US) | ✓ | |||
| Charcot-Marie-Tooth Disease | ✓ | |||
| Duchenne Muscular Dystrophy | ✓ | |||
| Idiopathic Pulmonary Fibrosis | ✓ | ✓ | ||
| Pneumonia | ✓ | ✓ | ||
| Bronchopulmonary Dysplasia | ✓ | ✓ | ||
| Tuberculosis | ✓ | ✓✓ | ||
| Type 2 Diabetes | ✓ | |||
| Diabetic Retinopathy | ✓ | |||
| Diabetic Neuropathy | ✓ | |||
| Diabetic Macular Edema | ✓✓ | ✓ | ✓✓ | |
| Age-related Macular Degeneration | ✓ | ✓ | ||
| Thyroid Eye Disease | ✓ | ✓ | ||
| Osteoarthritis (Cartilage Defects) | ✓✓✓✓ | ✓✓ | ||
| Post-operative Pain | ✓ | ✓ | ✓ | |
| Spinal Cord Injury | ✓ | |||
| Atopic Dermatitis | ✓ | ✓✓✓✓ | ✓ | ✓✓ |
| Erectile Dysfunction | ✓ | ✓ | ||
| Hepatitis C | ✓ | |||
| Graft versus host Disease | ✓ | ✓ | ||
| Hypertension | ✓✓ | ✓✓ | ||
| Bioequivalence, Medical Device | ✓✓ |
Pre-IND Projects: Buerger’s Disease, Traumatic Brain Injury, Bechet's Disease, Hunter Syndrome, Duchenne Muscular Dystrophy, Anthrax
Updated as of September 1, 2022


News & Events
KCRN Founder & CEO will be in South Korea until November 24, 2023 and will be available for any face-to-face meetings from any interested parties looking towards expanding into the US clinical research market.
Please contact us at info@kcrnresearch.com for any and all questions or inquiries! We hope to meet you soon!
(2023-10-20) KCRN Research working with non-profit Gfoundation to clothe underprivileged communities this winter.
KCRN Research is proud to announce that KCRN founder, Hugh Lee, is working with Gfoundation international non-profit organization based in South Korea to provide clothing to the underprivileged for the upcoming winter season and beyond.
https://gfound.org/bbs/board.php?bo_table=company_2022&wr_id=263&page=17
Gfoundation is a Christian non-profit that provides welfare and social development projects to underdeveloped communities in South Korea and globally around the world.
https://gfoundationus.org/
We thank Gfoundation for all the good works that they do in helping those in need as KCRN Research looks toward the second step planned to help more people this winter.
News & Events List
| Date | Subject | Contents |
|---|---|---|
| 2023-10-23 | KCRN CEO Hugh Lee will be available for face-to-face meets in South Korea | KCRN Founder & CEO will be in South Korea until November 24, 2023 and will be available for any face-to-face meetings from any interested parties looking towards expanding into the US clinical research market. Please contact us at info@kcrnresearch.com for any and all questions or inquiries! We hope to meet you soon! |
| 2023-10-20 | KCRN Research working with non-profit Gfoundation to clothe underprivileged communities this winter. | KCRN Research is proud to announce that KCRN founder, Hugh Lee, is working with Gfoundation international non-profit organization based in South Korea to provide clothing to the underprivileged for the upcoming winter season and beyond. https://gfound.org/bbs/board.php?bo_table=company_2022&wr_id=263&page=17 Gfoundation is a Christian non-profit that provides welfare and social development projects to underdeveloped communities in South Korea and globally around the world. https://gfoundationus.org/ We thank Gfoundation for all the good works that they do in helping those in need as KCRN Research looks toward the second step planned to help more people this winter. |
| 2023-10-20 | KCRN Research working with non-profit Gfoundation to help clothe underprivileged communities this winter. | KCRN Research is proud to announce that we are working with Gfoundation international non-profit organization based in South Korea to provide clothing to the underprivileged for the upcoming winter season and beyond. Gfoundation is a Christian non-profit that provides welfare and social development projects to underdeveloped communities in South Korea and globally around the world. |
| 2023-10-20 | KCRN is working with Gfoundation to provide clothing to the underprivileged this Winter. | KCRN Research is proud to announce that we are working with Gfoundation international non-profit organization based in South Korea to provide clothing to the underprivileged for the upcoming winter season and beyond. |
| 2022-10-07 | Hugh Lee of KCRN Research concluded a successful KSNS 42nd Workshop event in South Korea with a seminar presentation. | The Korean Society for Non-Clinical Studies 42nd Workshop titled - The Multiverse of New Therapeutics via Emerging Biotechnologies - has just concluded. KCRN Research and QuBEST BIO were presenters of the second day Luncheon Seminar #1. Thank you to all who attended! |
| 2022-09-02 | KCRN & QuBEST BIO at the 8th Annual KoNECT-MOHW-MFDS International Conference Oct 12 - 14 | KCRN and QuBEST BIO will be attending the 8th Annual KoNECT-MOHW-MFDS International Conference hosted jointly by the Korean National Enterprise for Clinical Trials, the Korean Ministry of Health and Welfare and the Korean Ministry of Food and Drug Safety. Come visit our booth and discuss with us about various tailored clinical solutions that fits you. Topic: Connecting Tomorrow Therapeutics to Patients Date: October 12 - October 14, 2022 Venue: Conrad Hotel, Seoul, South Korea |
| 2022-06-15 | KCRN & QuBEST BIO will be particpating in The 20th InterBiz Bio-Partnering & Investment Forum 2022 | Join us at our exhibitor booth at the conference in Jeju, South Korea! https://www.interbiz.or.kr/interbiz Meet experts in each field from both early development clinical CRO KCRN Research and non-clinical CRO QuBEST BIO to discuss the next stage of drug development and promote global clinical trials. < QuBEST BIO와 KCRN Research가 제20회 인터비즈 바이오 파트너링 & 투자포럼 2022에 참가합니다> https://www.interbiz.or.kr/interbiz/ ✔️ 비임상 전문 CRO QuBEST BIO와 초기 임상 전문 CRO KCRN Research가 함께합니다. ✔️ 각 분야의 전문가들을 만나 신약 개발의 다음 단계를 논의하고 글로벌 임상을 도모하세요 |
| 2021-11-18 | KSMS 40th Annual WORKSHOP Program of the Korean Society for Non-Clinical Testing and Research! | Date: December 16, 2021 Venue: Suwon Convention Center Convention Hall Topic: Dynamically Growing K-Bio Pharm in 2021 We welcome you to join us in the workshop event as we go over the successes that the Korean Bio and Pharma communities have already accomplished and discuss areas of needed improvement as well as further avenues for growth. Come stop by our booth and meet with us to talk about opportunities in your drug development plans. Our approach has always been to not just find the right solutions, but the right solutions for you. |
| 2020-10-12 | KCRN Hyungjoo (Hugh) Lee (CEO & Founder) presents a sharing seminar | KCRN Research CEO & Founder Hyungjoo (Hugh) Lee will be presenting a special virtual seminar this October 20, 2020 at 9 am Korea Time (Oct 19, 8 pm Eastern Time) titled 'Practical Aspects of Conducting Early Phase Clinical Trials in the United States' on the KIOSC Training online platform. Registration for this seminar is free and open to all who wish to gain a basic understanding of the processes that go into the critical early stages of clinical trials inside the largest market in the world. |
| 2020-10-05 | KCRN Research has new address | KCRN Research is relocating to a brand new home at 12311 Middlebrook Rd. Suite 200, Germantown, MD 20874. There are a lot of fond memories at our previous location where KCRN Research really grew as an organization, but it is also exciting to enter a new chapter for our company and see what the future holds for us. |
| 2020-09-16 | KCRN Presentation at KAPAL 2nd On-Air Webinar | Joseph Hong (Business Development) of KCRN Research spoke at the KAPAL 2nd On-Air Webinar that focused on the growing partnership between South Korea's and the state of Maryland's life science communities. The presentation included an introduction to the clinical CRO company as well as the opportunities that the Maryland life science environment has given companies like KCRN and for any others interested in doing business inside it. |
| 2017-03-27 | BioKorea 2017 International Convention | We have a plan to participate for three days (Apr 12-14, 2017) in the BioKorea International Convention with pharmaceutical and biotechnology companies. Please stop by at our booth to learn more about KCRN Research. KCRN Research will be available in South Korea Apr 10, 2017 to Apr 20, 2017 for meetings with Sponsors in Korea who are looking for the opportunity to develop drugs in the United States. We will be happy to consult with you to address your goals and the possibility of working with KCRN. Please contact Hugh Lee (hughlee@kcrnresearch, 301-540-2600 or 240-421-3190) or Jae Kim (jaekim@kcrnresearch.com, 240-277-9983) to make an appointment. |
| 2014-05-22 | 2014 Montgomery County Small Business Award | We are very proud to announce that KCRN Research won the Start-Up Business of the Year Awards at the 2014 Montgomery County Small Business Awards. We have come a long way since we started in 2012 and we would like to take this time to thank our partners and clients who have continued to put their trust in our business. Congratulations to the other winners at this event. For the full list of winners, please click here. |
| 2014-04-14 | 2014 Annual Korean Marketing Events | We are currently preparing for an event-packed summer in Korea to meet potential clients and promote the services that we have to offer. We hope to meet pharmaceutical and biotech companies who are interested in the KCRN way of early phase clinical development of world-changing drugs. 2014 Korean Society of Biochemistry and Molecular Biology (KSBMB) Annual Meeting We are attending the 2014 KSBMB Annual Meeting on May 15-16, 2014 at Booth #3 to meet with those interested in a cost-effective and efficient entry into early phase clinical trials. We hope to meet you there! BioKorea 2014 International Convention We look forward to spending a wonderful three days (May 28-30, 2014) in the BioKorea International Convention with pharmaceutical and biotechnology companies. Please stop by at our booth to learn more about KCRN Research. KCRN Research will be available in South Korea June 13, 2014 to July 12, 2014 for meetings with Sponsors in Korea who are looking for the opportunity to develop drugs in the United States. We will be happy to consult with you to address your goals and the possibility of working with KCRN. Please contact Hugh Lee (hlee@kcrnresearch, 301-540-2600 or 240-421-3190) to make an appointment. |
| 2013-10-23 | New Fall 2013 Marketing Campaign | Hello from KCRN Research! We would like to inform you that we have started a new marketing campaign featuring a variety of our clinical and regulatory services as well as competitive biotechnical and animal study services from two of our partnering biotechnology companies. With our collaborative efforts for a better quality and higher efficiency, we are proud to say that we make a wonderful package. Wherever you are on your journey to drug development, you will find that we offer the services that directly meet your needs. Our marketing campaign includes: KCRN Research (www.kcrnresearch.com) Practical and efficient clinical and regulatory services in early phase drug development from a sponsor’s point of view Curachem (www.curachem.com) High quality custom carbon-14 radiolabeling and stable isotope labeling to support your research and development programs Korean Institute of Toxicology (www.kitox.re.kr) Comprehensive and cost-efficient non-clinical safety studies to ensure a speedy and reliable entry into clinical trials For more information about each of our featured companies, contact us at kchoe@kcrnresearch.com or 301-905-8989. We will be happy to talk with you more about the particular services that may fit your company’s needs. |
| 2013-10-14 | Renovated website newly opened! | Hello from KCRN Research. As you may have noticed, we have completely renovated our website and updated all our posts! We are thrilled to say that our website now reflects KCRN more accurately. We are committed to effective communication. If you have any questions, please contact us at bd@kcrnresearch.com or 301-540-2600. Thank you for your interest in KCRN Research. |